Adult Efficacy
BAQSIMI demonstrated comparable efficacy to injectable glucagon in adult patients with Type 1 diabetes
Treatment success for BAQSIMI and glucagon injectable*

Treatment success was defined as the percentage of patients with either an increase in blood glucose to ≥3.9 mmol/L or an increase of ≥1.1 mmol/L from blood glucose nadir within 30 minutes.
Nadir is defined as minimum glucose measurement at the time of or within 10 minutes after glucagon administration. The mean nadir blood glucose was 2.5 mmol/L for BAQSIMI and 2.7 mmol/L for injectable glucagon.
*Study IGBC was a randomized, multicenter, open-label, 2-period, crossover study in adult patients with Type 1 diabetes (n=77) that compared the efficacy of a single 3 mg dose of BAQSIMI versus 1 mg injectable glucagon for treatment of hypoglycemia induced by intravenous insulin with a target blood glucose of <2.8 mmol/L. The primary efficacy measure was the proportion of patients achieving treatment success.
BAQSIMI began raising mean plasma glucose as early as 5 minutes*
Mean plasma glucose concentrations over time with BAQSIMI
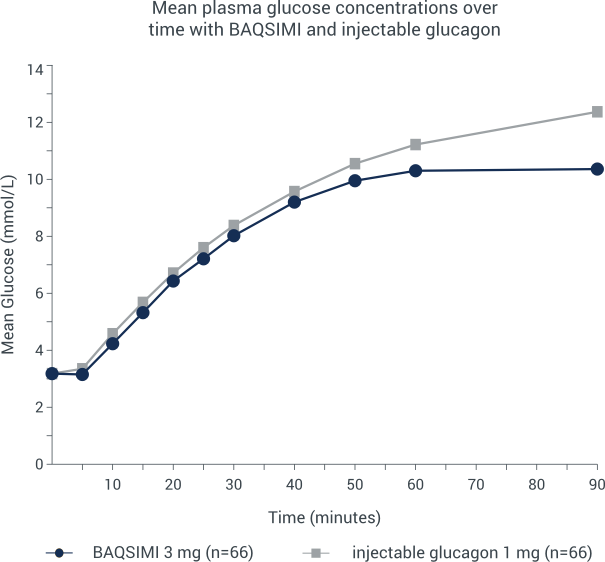
*A randomized, multicenter, crossover noninferiority trial was conducted in adult patients with Type 1 diabetes. Hypoglycemia was induced with intravenous insulin and treated by 3 mg intranasal glucagon or 1 mg intra-muscular glucagon. Plasma glucose was measured after each treatment was given.
Nasal congestion does not impact the absorption of BAQSIMI
